Chart a course for excellence with Fortrea FSP (Functional Service Provider)
Fortrea FSP excels in adapting to changing conditions and confidently navigating challenges. Through coordinated efforts and effective communication, our team will help you chart a course for success.
Global FSP success driven by people, relationships and innovation
With over 6,400+ staff across 70+ countries, Fortrea is one of the largest Functional Service Provider (FSP) CROs in the world. Our global infrastructure supports in-country, regional, on-, near- and off-shore models, each offering unmatched flexibility and scalability.
Backed by 120+ cross-functional relationships and a dedicated recruitment team, we bring deep expertise and proven capabilities to every engagement. Our commitment to innovation is reflected in our continuous investment in technology, enabling us to deliver efficient, high-quality solutions.
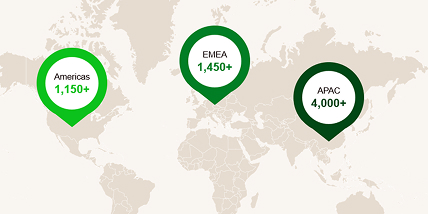
Fortrea FSP: Experience that matters
Delivering novel FSP solutions for 30+ years
Our story … starting with today
Our journey as an FSP innovator has been built through decades of experience.
Our FSP models are tailored to your needs, offering a range of FSP solutions and FSP services
FTE
- Available as staff augmentation or embedded line management
- Measures performance at the individual level
- Full FTEs
- Offers faster implementation and more customer control (but places more burden on customer)
- Provides FSP Teams customized to customers’ needs
- Focuses on recruitment and talent development
Centralized Delivery
- Task delivery is centralized to a delivery hub
- Leverages 24-hour workflow and offers high standardization
- Limited to services appropriate to centralization
- Examples include eTMF, trip report review and safety processing
Unitized
- Delivery is based on a task/work product as a unit, not by FTE (e.g., Data Management, Biostatistics and Statistical Programming)
- FSP option when Full FTEs not needed
- Examples include unblinded monitoring, long-term follow up monitoring, study start up (ICF and package review/approval)
Functional
- Single or multi-function
- Customer or Fortrea SOPs and/or systems
- Examples include Training as Service, Therapeutic Area or Country in a Box, Mobile Clinical Services, Regulatory Submissions, Data Management, Feasibility and Clinical Intelligence
Charting a course for excellence with Fortrea’s FSP team
Clinical Operations
Enhance the efficiency and effectiveness of clinical trials with Fortrea FSP. We deliver comprehensive clinical operations solutions and services encompass a full spectrum of delivery models, including FTE, unitized, centralized and hybrid approaches, tailored to meet the unique needs of our clients.
Our dedicated clinical operations team is committed to providing end-to-end support, enabling seamless execution and high-quality outcomes. With expertise in clinical monitoring, we deliver specialized oversight and management to maintain the integrity and safety of clinical trials. Fortrea provides specialized clinical services leveraging our global presence and extensive experience to drive success in clinical research.


Clinical Data Management
Improve the accuracy of clinical trials through our full range of clinical data management solutions. Our clinical trial data management services encompass end-to-end support, including project management, clinical programming and data validation. With a dedicated team of experienced professionals, we enable high-quality clinical data validation processes to maintain data integrity and compliance.
Our expertise in clinical monitoring and specialized clinical services, such as centralized medical data review and external data integration, allows us to provide tailored solutions that meet the unique needs of each study. Leveraging advanced technology and innovative approaches, Fortrea delivers reliable and efficient clinical data management solutions to support your clinical research.
Biostatistics and Statistical Programming
Fortrea's FSP Biostatistics and Statistical Programming services provide extensive biostatistics solutions and biostatistics programming services tailored to meet the needs of clinical trials. Our global team of experts provides clinical biostatistics services, including protocol design, sample size determination, statistical analysis plans and regulatory submissions.
We excel in statistical programming in clinical trials, utilizing advanced tools like SAS, R and Python to deliver robust statistical programming solutions. Our commitment to quality and innovation helps support our sponsors with precise and efficient data analysis, so we can serve as a trusted collaborator in the biostatistics and statistical programming landscape.


Safety Services & Medical Writing
Fortrea's "One Safety" approach offers a wide range of FSP safety solutions, providing robust drug safety and patient safety through advanced monitoring and pharmacovigilance services. With 34+ years of experience, our team at Fortrea excels in drug safety and pharmacovigilance—from clinical trials to post-approval stages.
Our dedicated Patient Safety Solutions (PSS) team, comprising 2,600+ safety experts, collaborates with global biopharma and medical device companies to enhance patient safety and regulatory compliance. Leveraging innovative approaches to pharmacovigilance, including automation and cognitive technologies, we deliver efficient and effective monitoring of patient safety, delivering life-changing medicines to patients faster.
Additional Fortrea FSP services
-
Medical Writing
Fortrea’s global Medical Writing team delivers end-to-end regulatory and medical communication support across all phases of clinical development. With 25+ years of experience, 1000+ documents authored and deep expertise across therapeutic areas, we offer flexible, high-quality solutions tailored to your program’s needs.
-
Mobile Clinical Services
We’re optimizing patient and site experience through our Home Nursing and Site Support Services. Learn how our global mobile clinical solutions bring trials to your patients—at home, at school or at work—to make the experience truly patient-centric.
-
Feasibility
Fortrea’s Standalone Feasibility services provides data-driven insights to optimize protocol design, country selection and site strategy—empowering sponsors with predictive, real-world evidence and global outreach. Led by therapeutically aligned experts, our tailored approach integrates proprietary analytics, local intelligence and stakeholder collaboration to accelerate study planning and enhance patient recruitment outcomes.
-
Regulatory Services
Fortrea offers critical regulatory support for clinical trial delivery, along with strategic guidance on registration, commercialization and implementation. Our regulatory strategy consultants can help you navigate complex regulatory processes and programs and keep your asset moving forward.
Our approach to charting a course for excellence with you

Making collaboration easy and effective
We structure our teams to embrace your unique organizational culture, operating systems and business needs.
By getting to know you and understanding your needs, we can rally our full range of expertise, capabilities and experiences to support you more effectively—making your job and your decisions easier.
Insights
Get the latest updates on Fortrea FSP
FSP-related frequently asked questions
Choose the level of oversight and accountability you need.
-
What is FSP in clinical research?
Functional Service Provider (FSP) in clinical research refers to our on-demand functional outsourcing model at Fortrea that supports one or multiple operational units—such as Clinical Operations, Monitoring, Data Management, Biostatistics and Statistical Programming, Pharmacovigilance, Medical Writing, Mobile Clinical, Feasibility and Regulatory.
-
What is the difference between FSO and FSP?
FSO (Full-Service Outsourcing) provides comprehensive support through multi-function clinical trial activities, from study design to regulatory submissions. FSO at Fortrea serves an end-to-end solution through holistic ownership of the entire clinical trial process in a seamless and integrated approach. Our FSO also integrates innovation and technology, benefiting from our advanced tools for data analytics, monitoring and other critical functions.
Our FSP (Functional Service Provider) services at Fortrea focus on delivering specialized expertise in areas such as data science, clinical monitoring and patient recruitment. Outsourced functionality allows sponsors to focus on core competencies relying on experts for specialized tasks and flexible resource allocation. Fortrea FSP also offers the ability to scale based on project requirements, optimizing efficiency and cost.
-
What is the difference between FSP and FSO models in clinical trials?
Our Fortrea FSP (Functional Service Provider) models involve outsourcing specific functions (e.g., data management, monitoring) while the sponsor retains control and oversight. With our FSO (Full-Service Outsourcing) models at Fortrea, we assume responsibility for the entire clinical trial process.
Overall, FSP offers more flexibility and integration with internal teams, while FSO provides end-to-end services.
-
Why are companies combining FSP and FSO strategies?
Hybrid models, which combine FSP and FSO approaches, allow our sponsors to tailor outsourcing to their needs—leveraging FSO for comprehensive services and FSP for specialized support. The FSP/FSO mix enhances efficiency, scalability and cost-effectiveness.
-
How does the FSP model support recruitment and talent development?
FSP companies specialize in sourcing, onboarding and training clinical research professionals. At Fortrea, we help sponsors overcome talent shortages by providing access to skilled professionals and maintaining high retention through dedicated support structures.
-
What role does the FSP model play in clinical operations?
Fortrea FSP streamlines clinical operations by embedding specialized teams within sponsor organizations. This model enhances agility, promotes compliance and supports faster decision-making through real-time collaboration.
-
How is the FSP model evolving to meet future clinical research needs?
Our industry’s FSP model is increasingly incorporating digital tools, AI and hybrid outsourcing strategies. FSP is also evolving to support decentralized trials, adaptive designs and global scalability while maintaining regulatory compliance.
-
What is the Follow-the-Sun model in FSP engagements?
The Follow-the-Sun (FTS) model enables 24/7 operations by distributing work across global teams in different time zones. This approach reduces turnaround times, enhances responsiveness and facilitates continuous progress in clinical development.
-
What are the benefits of using the Follow-the-Sun model in clinical trials?
Benefits of using the Follow-the-Sun (FTS) model include faster issue resolution, improved global collaboration and optimized resource utilization. FTS also supports real-time data access and enhances patient and site engagement across regions.
-
How does the FSP model address operational gaps in clinical trials?
Fortrea FSP provides targeted expertise and scalable resources to fill gaps in staffing, technology and process execution. Our service provides continuity, quality and efficiency in trial delivery.

















