Oncology Cell and Gene Therapies
Pioneering hope through your revolutionary cancer treatment
Harness our expertise in oncology cell and gene therapies (CGTs) to accelerate your cancer treatment breakthroughs. From early-phase trials to market access, we deliver tailored solutions that bring life-changing therapies to patients faster.
Specific oncology expertise
Leverage our deep experience in oncology cell and gene therapies, spanning diverse cancer types and treatment modalities.
Precision-driven oncology solutions
Harness advanced technologies and biomarkers to optimize patient selection, enhance efficacy and tailor trial designs specifically for oncology CGT.
Accelerated oncology breakthroughs
We progress oncology therapies with proven strategies that navigate complex regulatory landscapes and expedite clinical development.
Tailored solutions for your oncology CGT trials
Our specialists, versed in targeted therapies, immunotherapies and advanced CGTs, have pioneered biomarker-driven strategies that bring precision medicines to market. We bring our extensive oncology experience to every stage of the oncology clinical trial process to deliver the scaled capabilities that align with your study needs.
Across 19 countries and ~670 global sites, our experience in oncology cell constructs includes:
- 17 CAR T studies
- 2 TIL/γδ T cell studies
- 3 TCR studies
- 19 studies involving vaccines,
- TCEs, oncolytic viruses
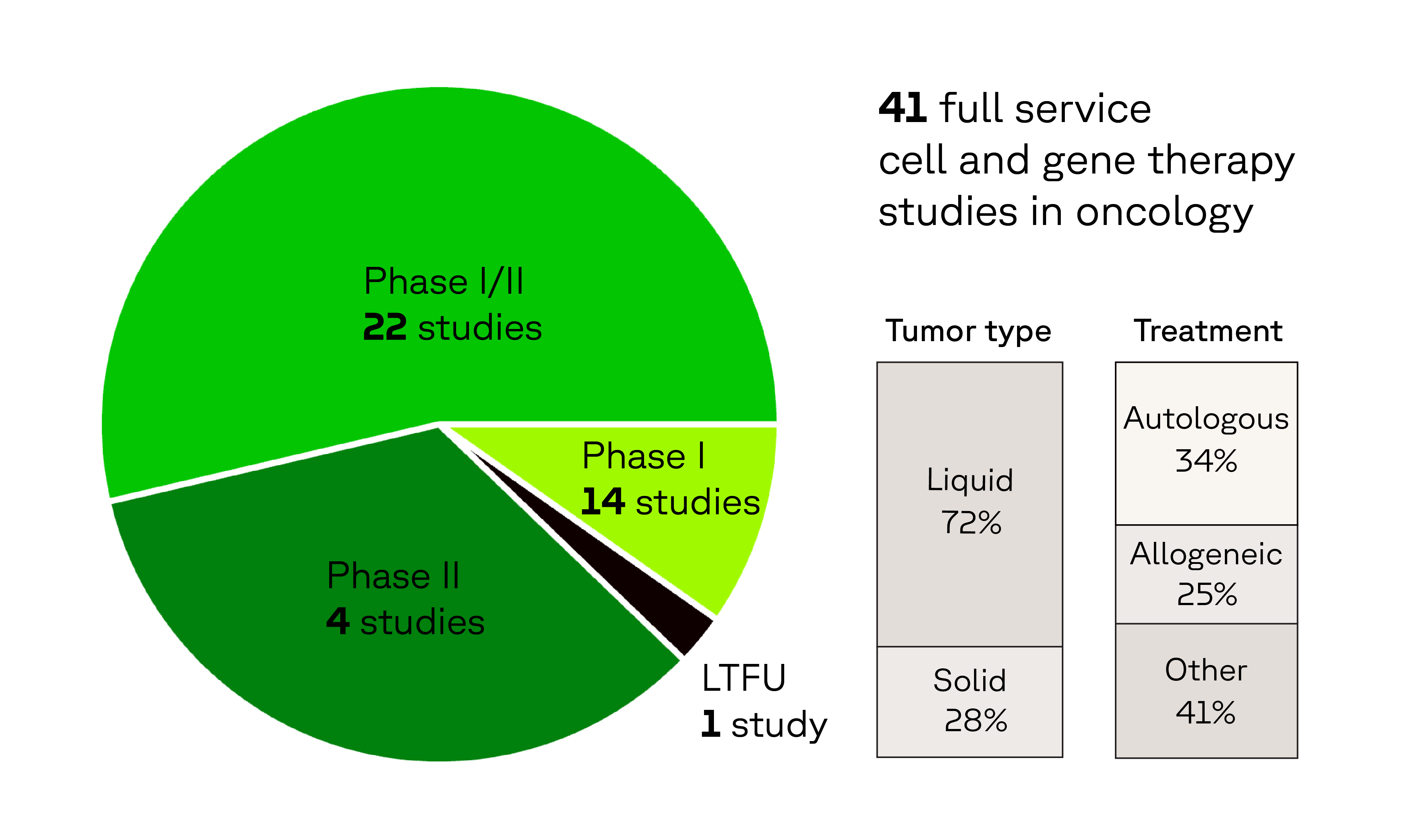
Oncology CGT experience that matters
In the last 5 years, we have supported:
Driving innovation in oncology CGT trials
Revolutionizing cancer care demands more than cutting-edge science; it requires innovative strategies, flawless execution and unwavering commitment. Our specialists apply forward-thinking approaches to navigate the complexities of these therapies, ensuring your trial not only meets but exceeds expectations.
-
Comprehensive CGT expertise
Our deep understanding of oncology cell and gene therapies extends beyond the science—we know how these complex treatments impact trial design and patients’ lives. Our support includes:
- Protocol development
- Apheresis
- Site training
- Complex cell therapy logistics
- Long-term follow-up (LTFU)
- Development of companion diagnostics
- GMO and ATMP global regulatory compliance
- Safety risk management
- Vendor qualification
- Medical monitoring
- Patient-centric burden mitigation
-
Optimized operational solutions
Oncology CGT development can involve asset-specific operational challenges that are reflected in the trial footprint. We bring flexibility, experience and comprehensive support to streamline your trial and bring hope to patients faster, including:
- Logistics support for autologous CAR T-cell therapies
- Site selection, activation, overall study setup
- Regulatory and GMO pathway time lines and approvals
- Site training for managing cytokine release syndrome, neurotoxicity, graft-versus-host disease
- Minimizing patient burden, including for LTFU studies
- Regulatory strategy design and implementation, with early engagement (e.g., INTERACT, pre-IND) for rare diseases and pediatrics
Related indications
Our deep experience in oncology coupled with specific CGT expertise help your trials run better.
Explore our related areas of expertise.

